ONE Lab LIMS
Digitalize all processes related to samples, tasks, and instruments.
More Than Just LIMS
With the laboratory being at the heart of every science-based organization, companies need to ensure smooth and efficient lab operations that are compliant with the different local and global regulations. While regulatory compliance and error reduction were the main drivers for the development of the first Laboratory Information Management Systems (LIMS), current requirements demand a more modern and agile approach to laboratory supporting systems than the large monolithic systems of the past.
BIOVIA ONE Lab digitalizes the processes related to the management of samples, laboratory tasks, studies and instrument metrology. ONE Lab integrates these systems and instruments together, leveraging a single data model, and automates the capture and transfer of data across lab operations and beyond. This unique architecture enables ONE Lab to take the place of a traditional LIMS solution, while also adding an integrated procedure execution layer.
Integrated Procedure Execution
BIOVIA ONE Lab digitalizes and integrates all of the data and processes in the lab, so that guided procedure execution is a seamless part of scientist's daily workflows. Test procedures are at your fingertips, with configurable tolerances, simple step-wise execution, and direct data capture.
Automated Review-by-Exception removes time-consuming manual approvals, accelerating batch release and increasing QC lab efficiency.
ONE Lab LIMS Software Components & Applications
BIOVIA ONE Lab brings truly seamless workflows to scientists in a unified environment, merging Lab Management, Procedure Execution, Materials Management and our Electronic Lab Notebooks. It provides a flexible, platform-based cloud solution that allows organizations to work in an agile cost effective manner while digitalizing and streamline lab workflows for regulated and non-regulated spaces. The future-proof approach allows organizations to orchestrate and control lab operations, manage and track samples and to easily adapt to changing needs.
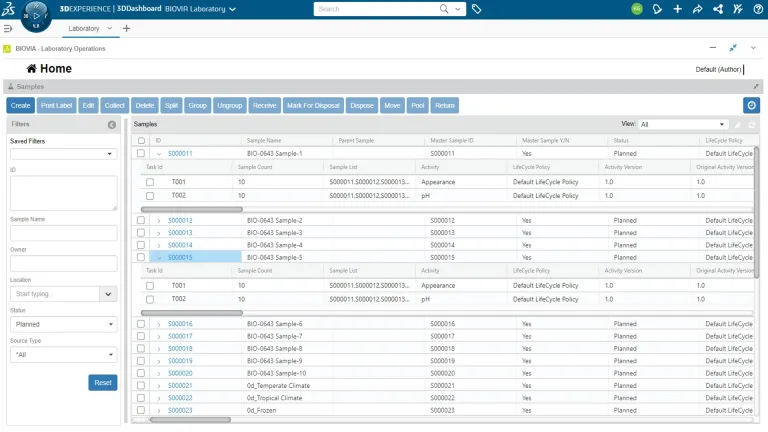
- Samples
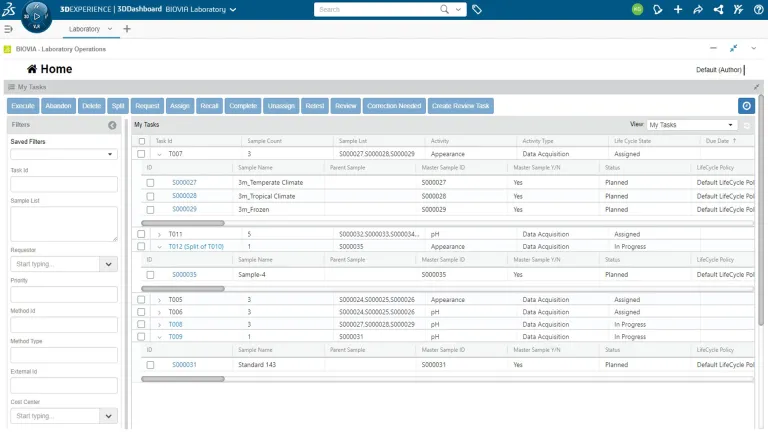
- Tasks
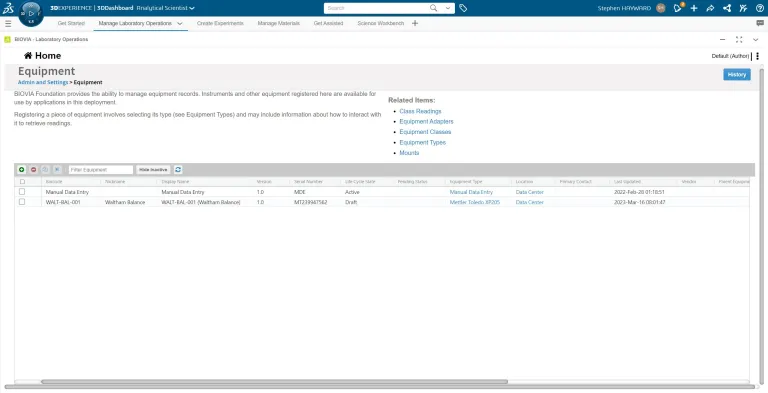
- Equipment
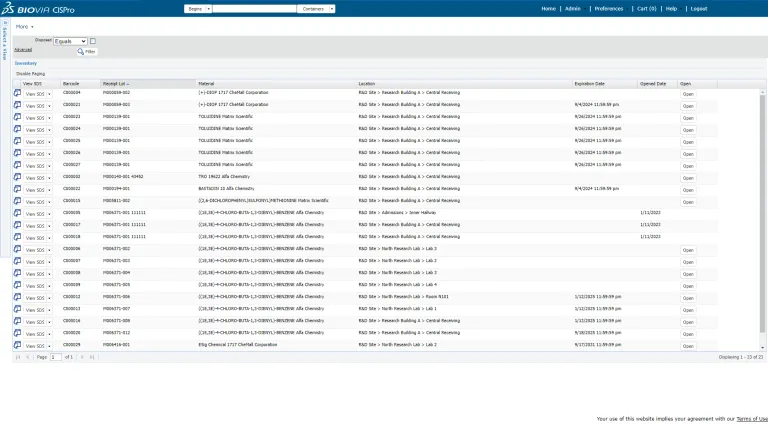
- Inventory
Sample Management
All aspects of sample management can be accomplished within ONE Lab, making it easy for scientists to keep track of their lab samples, and manage the flow of information throughout the sample lifecycle.
Task Planning and Management
In ONE Lab, scientists can easily create tasks, assign them to colleagues, and request their completion directly within a single electronic laboratory environment. Additionally, scientists can digitally manage the submission, routing, receiving, tracking and reporting of results originating from laboratory work requests and test orders.
Integrated Equipment Management
ONE Lab includes a comprehensive automated instrument data acquisition and the management of instrument -related data and workflows. Dedicated BIOVIA CDS Add-ins allow for bi-directional data exchange with Waters® Empower® and Thermo Fisher Scientific® Chromeleon®. Additionally, ONE Lab helps manage and track metrology events including required preventative maintenance, calibration and verification events.
Integrated Inventory Management
ONE Lab includes integrated chemical inventory management. Easily locate available consumables, and automatically track the amount used in laboratory tests. Increase safety by ensuring chemical storage is in compliance with local regulations. Gain visibility into materials usage at both the local and global level, and reduce waste.
ONE Lab LIMS Essentials
Get your new LIMS up and running quickly.
Bypass lengthy implementations and costly customizations with a pre-configured solution. Enjoy full LIMS functionality from day one and quickly achieve operational excellence.
Benefits of ONE Lab LIMS
- Increase efficiency through streamlined workflows
- Increase accuracy and consistency of data, information, and processes
- Reduce errors and re-work with automated data transfer
- Increase regulatory compliance though automation and standardization
- Preserve data integrity with centralized storage of standardized data across an integrated solution
- Reduce cycle times and costs by removing workflow bottlenecks
- Minimize IT overhead with web-based applications that require less IT resources and maintenance
Join the conversation in the BIOVIA Laboratory Informatics User Community!
FAQ about Laboratory Information Management System - LIMS
A LIMS (Laboratory Information Management System) is a software platform designed to manage and streamline laboratory operations. It helps labs track samples, manage data, automate workflows, and ensure compliance with regulatory standards. LIMS systems are commonly used in scientific and industrial labs to improve efficiency, enhance data accuracy, and support collaboration across different departments and locations.
LIMS (Laboratory Information Management System) is a software solution designed to manage lab workflows, sample tracking, data management, and compliance. TIMS (Test Information Management System) is typically focused on managing and tracking information related to testing processes, often in specific industries like manufacturing or quality assurance. While LIMS is more comprehensive for laboratory environments, TIMS is specialized for managing test-related data.
| Aspect | LIMS (Laboratory Information Management System) | TIMS (Test Information Management System) |
| Purpose | Manages laboratory operations, including sample tracking, workflow automation, and data management. | Focuses on managing and tracking information related to specific testing processes. |
| Scope | Broad scope, applicable to a variety of scientific labs like pharmaceuticals, biotech, and research labs. | Narrower scope, typically used in industries like manufacturing and quality control. |
| Functions | Covers sample tracking, data capture, regulatory compliance, and instrument integration. | Primarily focused on managing test-related data and ensuring test accuracy. |
| Industries Used | Used in industries like life sciences, healthcare, research, and environmental labs. | Common in industries such as manufacturing, automotive, and aerospace, where testing processes are critical. |
| Regulatory Focus | Ensures compliance with standards such as FDA, GLP, and ISO in lab environments. | Helps maintain compliance in quality control and testing, but less comprehensive than LIMS for regulatory needs. |
| Data Management | Comprehensive data management system, handling sample lifecycle, procedures, and results. | Typically handles only test-related data, focusing on the testing phase. |
| Aspects | LMS (Learning Management System | LIMS (Laboratory Information Management System) |
| Purpose | Software for managing and delivering educational content and training programs. | Software for managing laboratory workflows, sample tracking, and data management. |
| Primary Function | Focuses on e-learning, training, course management, and assessments. | Focuses on managing lab operations, including sample tracking, workflow automation, and compliance. |
| Users | Typically used by educational institutions, corporate training departments, and online course providers. | Used by labs in industries like life sciences, biotech, and environmental sciences. |
| Key Features | Course creation, assessments, user tracking, certification, and reporting. | Sample management, instrument integration, data storage, regulatory compliance features. |
| Industry Focus | Education, corporate training, and professional development. | Scientific research, pharmaceuticals, healthcare, and environmental testing. |
| Data Handling | Manages learning data such as course progress, assessments, and certifications. | Manages lab data such as sample lifecycle, testing results, and compliance documentation. |
There are several types of Laboratory Information Management Systems (LIMS), including:
- Enterprise LIMS: Designed for large organizations, offering extensive features for managing multiple labs and integrating with enterprise systems.
- Cloud-based LIMS: Hosted on the cloud, providing flexibility, scalability, and lower maintenance costs.
- Industry-specific LIMS: Tailored to meet the unique needs of specific industries like pharmaceuticals, biotechnology, or environmental labs.
- Modular LIMS: Offers customizable modules that allow labs to add features as needed, providing flexibility in system configuration.
ONE Lab LIMS software enhances regulatory compliance by automating the documentation and validation of lab processes, ensuring adherence to industry standards such as FDA, GLP, and ISO. It provides features like audit trails, electronic signatures, and controlled data access, all crucial for maintaining compliance with local and global regulations.
Also Discover
Learn What BIOVIA Can Do for You
Speak with a BIOVIA expert to learn how our solutions enable seamless collaboration and sustainable innovation at organizations of every size.
Get Started
Courses and classes are available for students, academia, professionals and companies. Find the right BIOVIA training for you.
Get Help
Find information on software & hardware certification, software downloads, user documentation, support contact and services offering