ONE Lab Compose
Standards-Based Procedure Management and Authoring
Improve Lab Efficiency with Standardized Procedures
Today’s science-based industries are challenged to quickly and efficiently transform new product development processes and analytical methods into reliable and repeatable procedures. Ultimately, any time reduction in the product commercialization process not only reduces investment cost, but shortens the time to which a return on investment is realized.
One manner in which to directly address this challenge is by implementing a standards-based approach to product development and scale up, by driving utilization of a recipe/method framework from the early stages of development. A procedure could represent any number of processes, such as a synthetic chemistry or formulation recipe, an analytical method, a standard operating procedure, or a protocol. Ensuring consistency in procedures reduces re-work, streamlines workflows, standardizes data, and removes barriers in tech transfer to manufacturing.
Easily Build Complex Methods
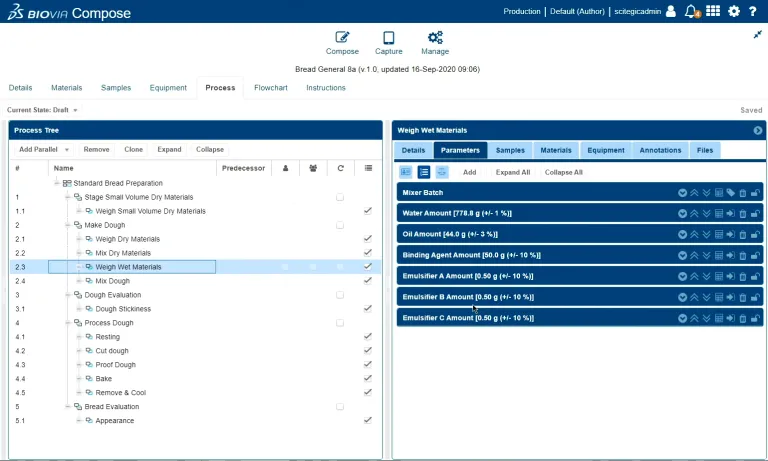
In BIOVIA ONE Lab, scientists create methods from libraries of procedures and activities, using a drag-and-drop interface. Unique procedures are easy to define and add to the process library. Complex parallel and inter-dependent procedures can be built and visualized with the process tree, with definitions for stepwise limits and instructions. Materials and equipment management are also integrated, allowing scientists to define the specific types of available equipment and materials to be used in the authored procedures and recipes.
Simplify Tech Transfer
Procedures authored in ONE Lab leverage the ANSI/ISA-88 standard, ensuring consistency as methods are transferred from product development to the scale-up and manufacturing phases. No longer do procedures need to be re-created from scratch in each new system, instead relying on 4 well-defined layers (process, stage, operation, and action) to accurately describe all lab activities in a way that batch production systems can recognize.
- Capabilities
- Benefits
Procedure Authoring Capabilities
- Create procedures for your enterprise using built-in libraries of operations and actions
- Be flexible with a dynamic web-based user experience on desktop, laptop and mobile devices
- Integrate with material and equipment management systems
- Define parallel operations and dependencies
- View Process Instructions and Process Flow
- Externalize and transfer procedures via S88 standard
Procedure Authoring Benefits
- Standardization with an integrated solution – from laboratory procedure development to execution on the manufacturing plant floor
- Streamlined global tech transfer from bench to pilot plant
- Strengthened adherence to regulatory, safety and QA requirements
- Transparency across functional lines on past, current and future development projects
- Systematic re-use of organizational knowledge, experiences and best practices
Also Discover
Join the conversation in the BIOVIA Laboratory Informatics User Community!
Learn What BIOVIA Can Do for You
Speak with a BIOVIA expert to learn how our solutions enable seamless collaboration and sustainable innovation at organizations of every size.
Get Started
Courses and classes are available for students, academia, professionals and companies. Find the right BIOVIA training for you.
Get Help
Find information on software & hardware certification, software downloads, user documentation, support contact and services offering