Mobilization around healthcare
The transition to genuine precision medicine will transform the way we carry out research and the way we think about data.
A new dawn and mobilization around healthcare
Although MEDIDATA initially focused on processing data produced by clinical trials, we also use data from other sources. In 2020, hundreds of millions of patients and thousands of researchers had active, simultaneous access to more than 30 terabytes of raw data, generated from sources that are updated daily, within the MEDIDATA environment. The system uses billions of documents, and we are proud to be part of this new era for the whole of society and the mobilization around healthcare in general.
New methods and tools will emerge in all therapeutic areas. Before COVID-19, everything worked on the assumption that the caregiver and patient would be present in the same room. That’s what happens when a patient volunteers to take part in an on-site clinical trial or, even more frequently, when a patient consults his or her doctor. But this was not the right assumption. We are now seeing a wave of innovation around virtual trials and new ways of thinking about research.
The transition to genuine precision medicine will transform the way we carry out research and the way we think about data.
Precision medicine originated in the field of oncology, based on analyzing the molecular and genetic characteristics of tumors. Research has shown how genetic damage occurs within normal cells, triggering the processes that lead to cancer, which vary from patient to patient. Progress with genome sequencing has allowed us to develop molecular tests and therapies that target these cancer-causing phenomena. These are examples of a convergence of biological and digital domains.
However, precision medicine creates a paradox. Precision medicine means, almost by its very nature, that there will be a decreasing number of patients benefiting from the therapies it produces. The more precision we achieve, the more closely we define the patient population, the more data we need to collect, and the harder it is to collect them and generate the evidence required.
The transition from therapies based on large cohorts to genuine precision medicine will transform the way we carry out research and the way we think about data. And when I talk about convergence, I’m also thinking about how we can represent the various therapies on a Venn diagram, with one set corresponding to molecules, another to medical devices and another to digital therapies. Convergence means that the intersections between these various sets will increase.
Finally, we must ask questions about access to care: How do we offer therapies to patients in a fair way, regardless of their socioeconomic circumstances and geographical situation?
Glen DE VRIES, Co-founder and co-CEO, Medidata
More than 23,000 clinical trials have already been carried out using MEDIDATA solutions
More than 40% of clinical trials around the world rely on MEDIDATA solutions
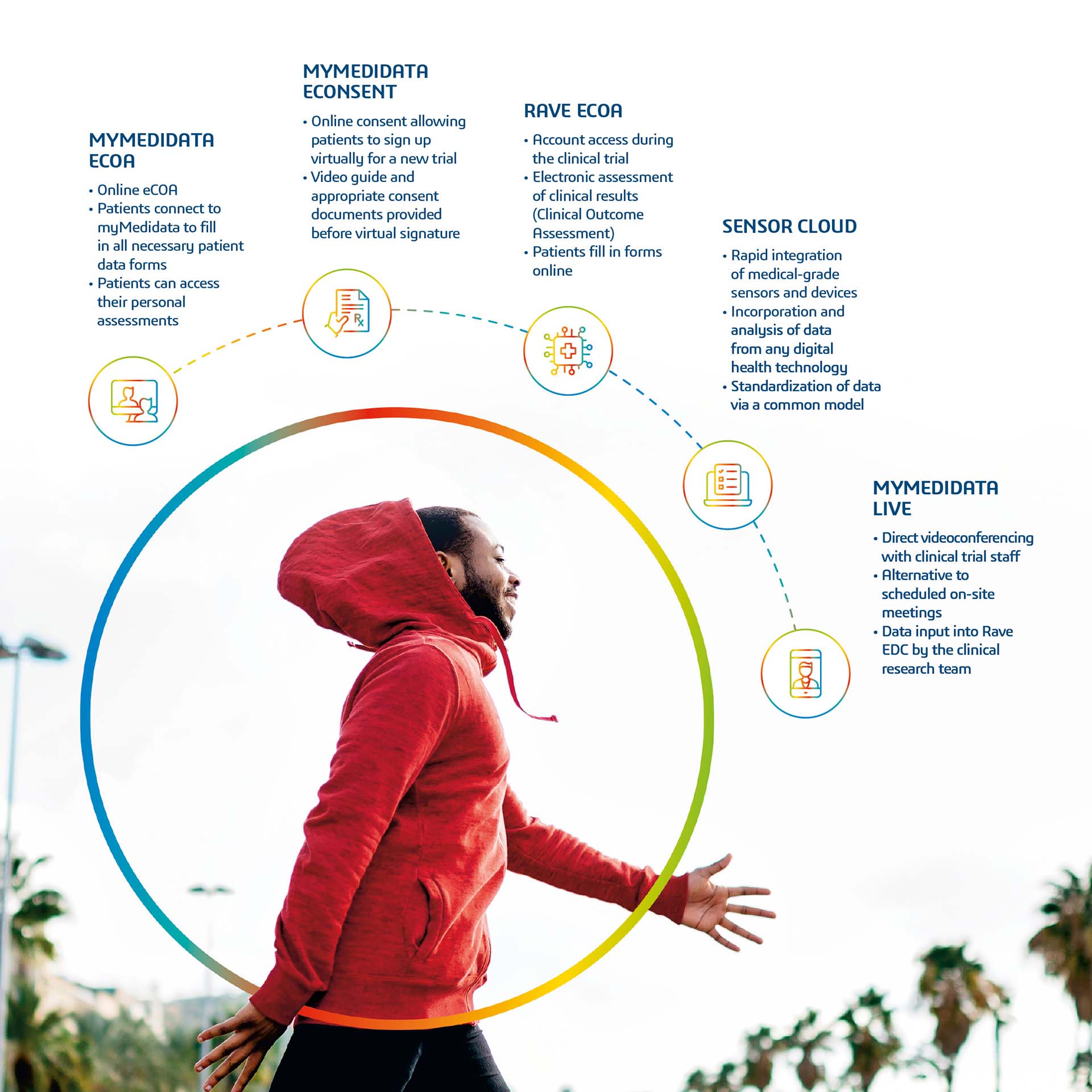
PATIENTS PLAY AN ACTIVE ROLE IN CLINICAL TRIALS WITH MYMEDIDATA
myMedidata is a new platform that can acquire data from various sources, from treatments as well as from direct interactions with practitioners, and so can play a major role in patient-centric research. myMedidata comprises all Patient Cloud tools and the Rave platform, both of which comply with current regulations. myMedidata also includes eConsent, an electronic system for obtaining patients’ consent to take part in clinical trials; eCOA (electronic Clinical Outcome Assessments), a tool for assessing the results of clinical trials; ePRO, which is used to integrate results obtained electronically from patients; wearable sensors, which collate data collected by biosensors and wearable tech and virtual trials.