The Virtual Twin Experience for Biomanufacturing
Disruption, innovation, and global connectivity are all reshaping how biomanufacturers deliver today's novel therapies - the virtual twin experience can help you transform your operations.
The Life Sciences industry has changed significantly over the last number of years. With a view to developing new, novel, more effective, quality, precision treatments, industry leaders are exploring new therapeutic areas and approaches, leveraging the latest technology advancements to gain the competitive edge and deliver sustainable scientific innovation.
As pharmaceutical manufacturing lines transition towards small batch production to produce precision medicines, manufacturers need to effect end-to-end manufacturing line optimizations to produce these therapies more sustainably. To do this, manufacturers look to connect systems, people and data — leveraging digitalization, automation, virtualization, modeling and simulation and Artificial Intelligence (AI) and what we call the Virtual Twin. The Virtual Twin, is a digital replica of real-world pharmaceutical processes, products and plants from end-to-end. When leveraged with a platform based approach, it delivers the agility manufacturers need with science-based tools that can be leveraged to develop novel therapies. Explore more about the Virtual Twin for BioManufacturing in the chapters below.
01 Resource Planning
Coordinate all plants to optimize full planning activities with the supply chain Virtual Twin experience. Using “What-if” scenarios, compare and optimize planning based on world-class KPI optimizations and experience increased flexibility enabling adaption to demand fluctuations to ensure ontime delivery.
Explore Resource Planning Solutions
- Sales & Operations Planning
- Master Production Scheduling
- Production Scheduling
- Quality Control
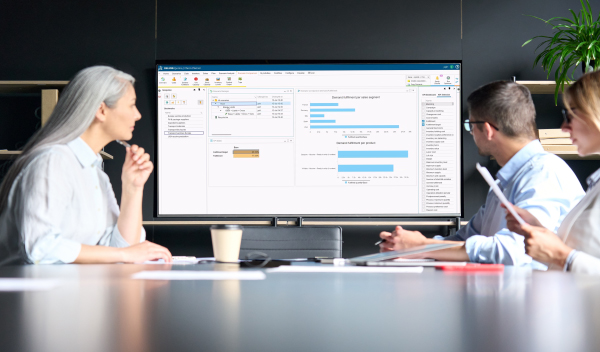
Sales & Operations Planning provides a complete workflow and supports the full process of Sales & Operations - predicting future drug demand and optimizing the supply chain based on the forecasted demand input.
Companies can;
- Better forecast demand to deliver drugs on time to patients
- Optimize supply contract considering the full value network: suppliers, CMOs & wholesalers
- KPIs driven S&OP Execution for a better drug operational management
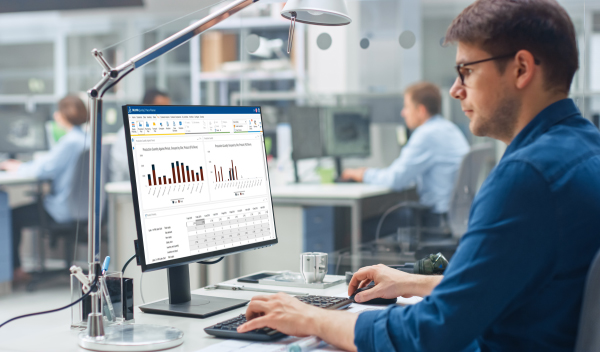
Master Production Scheduling empowers pharmaceutical manufacturers to reduce drug shortage tension in the supply network. Wholesalers can be supplied on time with high delivery performance and profitability by streamlining and simplifying complex tactical planning decisions.
Companies can:
- Optimize overall drug volume delivery
- Perform constraint based finite capacity planning considering the network (Plants / Internal)
- Ensure adequate material supply throughout the plant to satisfy the demand at the right time
Production Scheduling provides integrated management of product and process-related constraints for detailed production scheduling across multiple resources: machines, operators, tools and metrics in the fast-moving twenty-first century biopharmaceutical manufacturing environment.
Companies can;
- Manage demand per product for agile Production
- Leverage strategic, tactical, inventory and capacity planning
- Schedule operations and optimize the plan through simulation
- Visualize and analyze scheduling information to make fast decisions
QC Lab Scheduling provides integrated management of resources and product-related constraints for detailed scheduling across people, instruments, equipment and tasks in accordance with availability, training, skills and readiness.
Companies can;
• Manage sequencing for agile QC laboratories
• Create short and mid-term strategic, tactical, inventory and capacity plans
• Visualize and analyze scheduling information to make fast decisions
02 Build & Simulate
Define, develop, optimize and validate recipes and the associated industrial setup by building the Virtual Twin of the manufacturing environment. Improve production flexibility with “What if Scenarios” to simulate, and analyze multiple production and process engineering scenarios, in a realistic 3D environment, optimizing the production workflow and resource utilization.
Explore Build & Simulate Solutions
- Recipe Management
- Process Engineering
- Line Model & Simulation
- Line Qualification & Commissioning

Recipe Lifecycle Management allows to author, test and release new recipes faster using lifecycle management capabilities and therefore reduce time to market and ease recipe development in a sustainable manner.
Companies can:
- Support the industrialization of recipes and their smooth transition to execution.
- Access vertical knowledge and know-how about equipment and process
- Assess environmental metrics of the complete and configured manufacturing definition
Line Process Engineering allows to define, simulate and validate the behavior of complex multi-physics systems (Piping & Tubing, Air Conditioning and Electrical) and ensure engineering integrity through a system engineering and model-based design approach.
Companies can:
- Efficiently define and decompose systems architecture
- Develop and manage your engineering data through model-based schematic representation
- Rapidly model, simulate and validate complex engineering systems
- Integrate Control Systems and 3D Product Design Processes to Leverage Intelligent Control Systems
With Line Model Design and Line Simulation & Optimization, you can synchronize all engineering processes & simulate and optimize the dynamic behavior of production scenarios to derive a performance-driven, ergonomic plant layout.
Customers can:
- Quickly develop layout, conceptual building information models and evaluate design decisions
- Create accurate and flexible 3D system design
- Manage shop floor layouts through a collaborative and intuitive 3D Mock-Up environment
- Simulate manufacturing processes, equipment and human tasks before the production line even exists
With Line Virtual Commissioning, you can simulate and create a virtual twin of your entire system, which can be tested and validated even before being implemented in the real physical plant, thus, saving you time and money.
Customers can:
- Test and validate control systems of pharmaceutical production lines on its virtual twin before it even exists
- Support high level test process including generation of GAMP compliant documentation for the development of the simulation model
- Iterate quickly together to find the best alternative & reuse the simulation model as Operator Training Systems for simulation of stress situations
03 Operate & Control
Effectively manage and achieve manufacturing operational excellence with the Virtual Twin experience. Unify manufacturing operations with warehouse processes, and experience full continuity between recipe authoring and recipe execution on the shop floor and in QC laboratories to minimize waste. Quickly adapt and scale up the production with fast refitting of processes and layouts to deliver flexibility and improved overall equipment effectiveness.
Explore Operate & Control Solutions
- Lean Management
- Quality Control Lab Execution
- Material Synchronization
- Modular Manufacturing
Lean Operational Management offers the ability to digitalize Lean Practices on the Shop-Floor. By leveraging big touch screens technologies, it enables Lean representatives to broadcast company Lean Practices and ease the animation of Shop-floor meetings between team leaders, its team and other support functions. 3Dlean helps in capturing and retaining institutional knowledge and intellectual property and manufacturing know-how.
Customers can:
- Digitize Lean Practices for your operational meetings
- Deliver full empowerment to your team
- Create Digital Continuity across organizational barriers
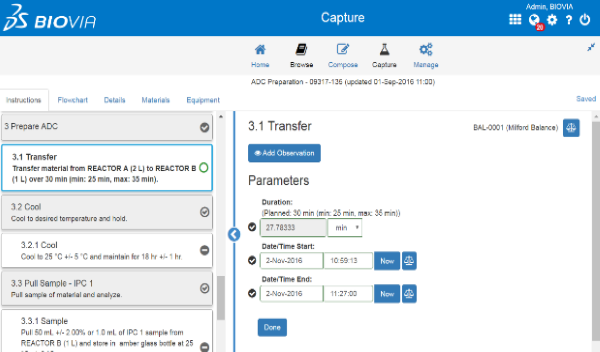
Quality Control Lab Execution is a compliant solution to schedule and document workflows and outcomes for raw material, finished product, in process quality control testing.
Customers can:
- Digital process to manage samples and resources, task scheduling, method execution and results review.
- Industry standard S88 documentation and execution of methods.
- Standardization of Quality Control laboratory activities.
Material Synchronization tightly unifies manufacturing operations with warehouse processes. It directs people and equipment to perform warehouse activities, such as put-aways and cross-docking materials directly to and from production. Material synchronization synchronizes material flows with manufacturing operations to support Just-in-Time, Lean and other continuous process improvement initiatives.
Customers can:
- Monitor logistics activity and inventory levels in real-time
- Fully integrates production line requirements with warehouse activities
- Monitor pharmaceutical plant logistics activities in real-time
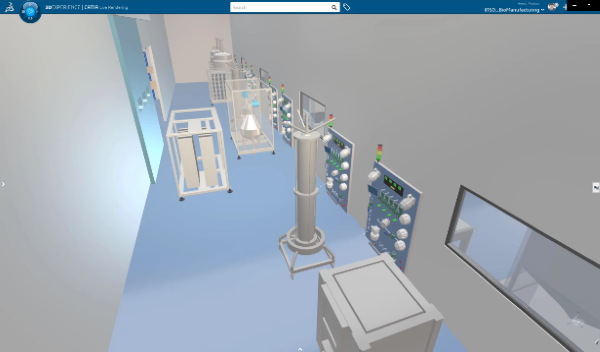
Pharmaceutical manufacturers can support modular manufacturing using the 3DEXPERIENCE platform to produce a variety of products from a single facility with faster changeovers, increased flexibility, and improved overall equipment effectiveness.
Customers can:
- Ease recipe scale-up by developing, testing and releasing recipes faster
- Achieve more agile and flexible manufacturing for changing demands through fast layouting
- Simulate the behavior of processes, human activities and production lines before the line even exists
Learn how Sanofi produce better vaccines faster with modular manufacturing.
04 Optimize & Predict
Experience process control and optimization insights based on simulation, real time data gathering and data prediction with virtual twins. Understand and monitor the process to improve quality, sustainability and increase yield. Ensure safe batch release by monitoring production to identify out of control trends before failures occur and provoke waste of materials.
Explore Optimize & Predict Solutions
- Batch Process Analytics
- Environment Impact Simulation
- Sustainability
Batch Process Analytics ensures continuous production by identifying critical process parameters and quality attributes and monitoring production to identify out of control trends before failures occur.
Customers can;
- Minimize non-value-adding manual tasks,
- Reduce the risk of errors and compliance,
- Promote process understanding and knowledge sharing to reduce process variability.
Build Environment Performance Analysis allows virtual analysis of airflows in buildings to ensure safe working environments and improved compliance with industry air quality regulations
Customers can;
- Simulate natural & forced air flow paths & potential direction of contamination
- Imagine multiple process design scenarios to comply with regulations requirements
- Assess propagation/contamination risks in working areas

To meet the high demands of sustainability, traditional ways of innovating will not affect manufacturing sustainability quickly or effectively enough. Instead, manufacturers need to leverage the power of the virtual twin to optimize their real-world impact before building their plants and supply network.
Customers can;
- Realize the agility manufacturers need with science-based tools to develop sustainable novel therapies.
- Improve old and non-sustainable operations
- Limit pharmaceutical manufacturing impact on human health, ecosystems and resource availability through Lifecycle Assessment
Discover More
Browse our articles and assets to discover more information on how the virtual twin experience can transform your biomanufacturing operations.
Explore our Biopharmaceutical Solutions Experiences
Learn more about how our Industry Solutions can help you achieve your professional ambitions and business objectives.
