BIOVIA Structured Document Authoring
Transforming Document Authoring
Understanding CMC Regulatory Requirements
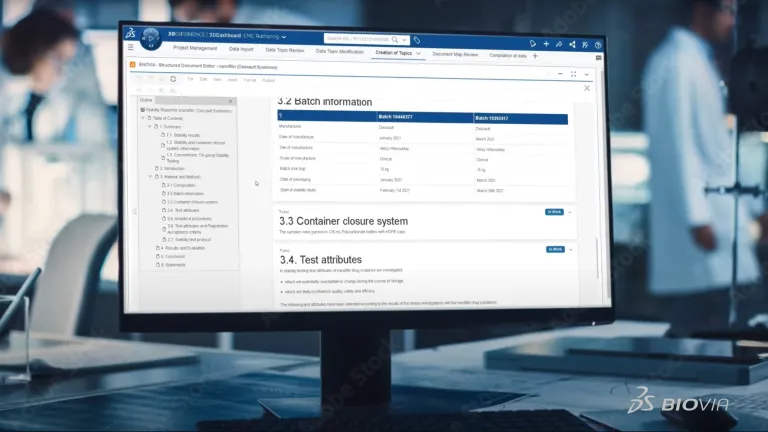
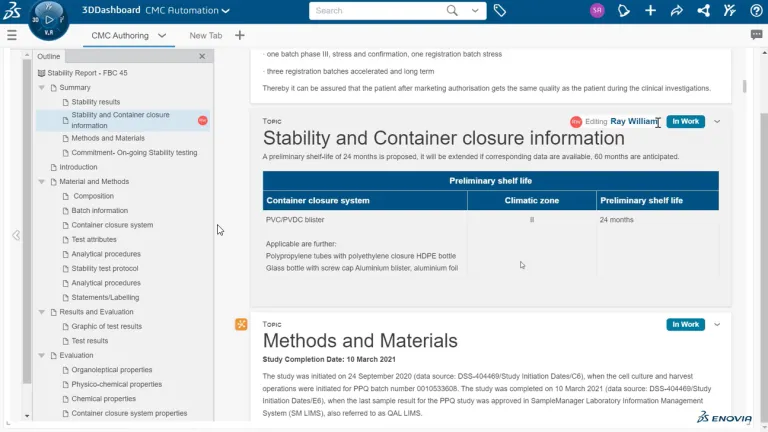
CMC (Chemistry, Manufacturing, and Controls) regulatory documents are a critical part of pharmaceutical submissions to regulatory bodies such as the FDA and EMA. These documents provide detailed information on a product’s manufacturing process, quality controls, and stability to ensure safety and efficacy. Structured document authoring streamlines the creation and management of CMC regulatory documents, reducing errors, ensuring compliance and speeding time to market.
Tackling the Challenges of CMC Dossiers
The creation of scientific and quality documents is often complex, cumbersome and time consuming. Manual data entries must to be re-verified. Multiple experts across the organizations have to collaborate to compile large amounts of data to create documents of hundreds of pages like for CMC dossiers. And furthermore, document creation is not a one time off. It is an ongoing process. Some of them are produced over ten to fifteen years of product development time and still updated.
Are you still creating your CMC dossiers via copy-and-paste and reverifying every single data point? Then your processes might be error-prone, leading to inconsistencies, lacking traceability and endanger your IP. BIOVIA Structured Document Authoring is a radically new way to create documents. It is data-centric moving away from static documents. It is automated versus manually searching and entering data. Which also make data reverification obsolete.
Capabilities of Structured Document Authoring
- Author and share documents
- Standardize document content, format and layout
- Automatically update document content
- Collaborate securely and controlled
- Get oversight of updated and used content
Key Benefits of Structured Document Authoring
- Reduce time creating dossiers by 80%
- Reduce costs by $2M+ per dossier
- Produce accurate reports with current and up-to-date data
- Save 30 – 70% of time omitting data reverification
- Maximize transparency and traceability
- Protect your IP
- Speed time to market
Start Your Journey
The world of Structured Document Authoring is changing. Discover how to stay a step ahead with BIOVIA
FAQ About Chemistry Manufacturing and Control
Also Discover
Learn What BIOVIA Can Do for You
Speak with a BIOVIA expert to learn how our solutions enable seamless collaboration and sustainable innovation at organizations of every size.
Get Started
Courses and classes are available for students, academia, professionals and companies. Find the right BIOVIA training for you.
Get Help
Find information on software & hardware certification, software downloads, user documentation, support contact and services offering