BIOVIA CISPro
Chemical and Material Inventory Management
Right Chemical. Right Place. Right Now.
The daily function of laboratories across industries revolves around the available chemical inventory. Chemicals are the raw materials for most experiments, holding the key to research innovations, and managing the inventory efficiently can make or break a laboratory’s budget.
Outdated chemical inventory systems can result in long searching times, either too much or too little material on hand, leading either to excess waste or delays in experimentation schedules. Managing and ordering chemicals with these systems can be a tedious and time-consuming process. Additionally, maintaining a comprehensive overview of the types and amounts of hazardous materials on each site can become difficult and complex. To decrease the risk of incidents, increase efficiency, and lower costs, a unified digital chemical inventory management system is the answer.
For large companies with many users in multiple facility types and locations, BIOVIA CISPro delivers a complete and sustainable solution that allows you to utilize existing workflows, yet streamline your chemical and material inventory management processes.
Key Benefits
- Reduce costs with fewer duplicate orders, and better management of expiring inventory
- Increase efficiency through streamlined workflows, easier location of inventory, and faster report creation. Spend up to 75% less time searching for inventory.
- Increase regulatory compliance with harmonized inventory management across global sites, fewer errors, and GxP compliant workflows.
- Reduce risks with a better overview of hazardous and flammable materials stored on each site, and better quality of data and documentation
A Leader in Chemical Inventory Management
- Capabilities
- Flexible Deployment
- Lab Integration
BIOVIA CISPro Capabilities include:
- Real-time chemical and material inventory data from receipt to disposal
- Standardize procedures and track materials across multiple sites
- Manage chemicals, biological materials, and supplies on container level in one place
- Pre-populated vendor catalogue to streamline data entry
- Multi-site, multi-tier location hierarchy
- Complete audit trails for regulatory compliance
- Integrate chemical inventory with SDS
- Automated Tier II, Fire Code, Title 19 reporting
- Specialized modules for hazardous materials identification, controlled substances, regulatory list management, biological materials management, and SDS management.
On Cloud or On Premise
BIOVIA CISPro can be deployed on the cloud, using the infrastructure of BIOVIA ScienceCloud, in either a public cloud or validation-ready private cloud environment. Cloud deployment minimizes IT overhead and ensures security procedures are carried out by dedicated professional security experts. CISPro can also be deployed and validated on-premises if desired.
Integrated with BIOVIA ONE Lab
BIOVIA CISPro seamlessly integrates with the rest of BIOVIA ONE Lab, including our ELNs, sample management, task planning, and procedure execution workflows. This powerful combination lets organizations integrate BIOVIA CISPro with their lab instruments and other data sources while also providing rich capabilities for scientific analytics, visualization and automated report generation.
Detailed Inventory Tracking
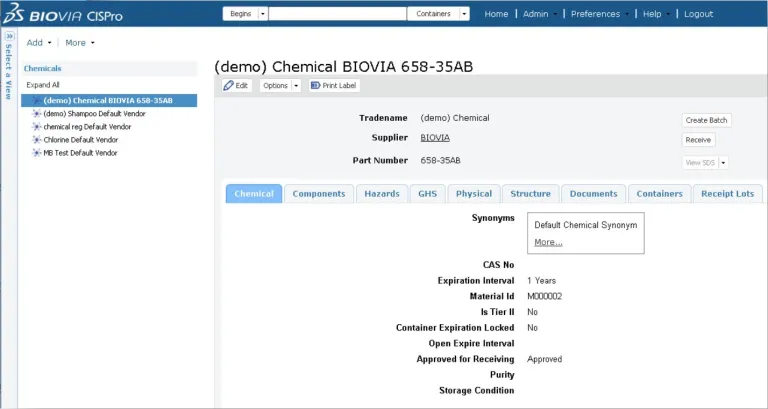
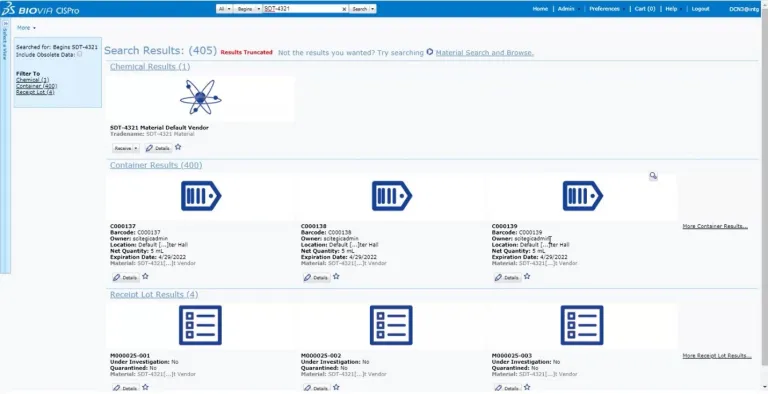
BIOVIA CISPro enables organizations to maintain a listing of all the chemicals and materials in each facility at the container level, keep track of where they are in real-time and monitor usage. An unlimited number of material classes can be tracked, including reference standards, with multiple security layers. Inventories for individual business units can be maintained independently, yet managed collectively under the same company account.
BIOVIA CISPro delivers all the necessary tools to accurately track and report chemicals and supplies including controlled substances while meeting safety and regulatory requirements, including barcode labeling, remote inventory control and Safety Data Sheet (SDS) management. Reports are easy to generate, allowing chemicals to be listed by location, vendor, name, CAS#, formula, etc. Most importantly, hazard information is always easy to access during an emergency.
Global and Local Regulatory Compliance
For multinational companies, safety data can be configured and managed based on country or region, in multiple languages, and the system can generate required regulatory reports to meet any regional requirement.
The web-based delivery of BIOVIA CISPro simplifies implementation, eliminating the need to install software on every user's computer, while the system’s ability to operate on low bandwidth connections ensures ease of operation anywhere in the world.
FAQ About Chemical Inventory Management Software
Also Discover
Learn What BIOVIA Can Do for You
Speak with a BIOVIA expert to learn how our solutions enable seamless collaboration and sustainable innovation at organizations of every size.
Get Started
Courses and classes are available for students, academia, professionals and companies. Find the right BIOVIA training for you.
Get Help
Find information on software & hardware certification, software downloads, user documentation, support contact and services offering